In the previous article, I explained that a nucleus is made of protons and neutrons. One important difference between protons and neutrons is the electric charge. A proton has a positive electric charge while a neutron is electric charge neutral.
But doesn’t this strike you as a little strange? Protons carry a positive charge, while neutrons carry no charge. It’s intuitively clear that two particles with the same charge would repel each other. So why, then, can atomic nuclei exist stably?
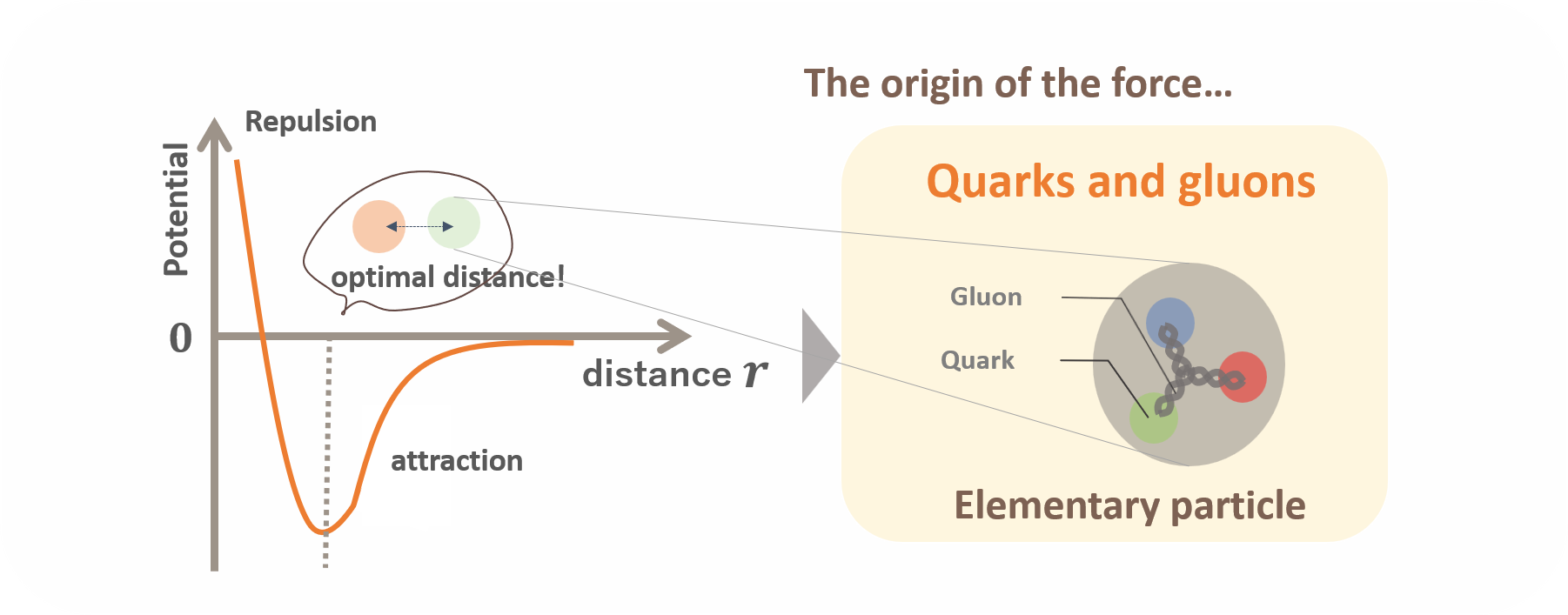
Here come the quarks and gluons. Why can positively charged protons and electrically neutral neutrons coexist stably? It is because nucleons, in other words protons and neutrons, are bound together by the “strong force”. Although we feel or see electromagnetic force and gravitational force in our daily lives, the strong force is another type of force beside them. It only works at extremely short distances, such as within the small small atomic nuclei, so we do not perceive it in our everyday experiences. However, it is a far stronger force than either electromagnetism or gravity. For the sake of this strong force, all the matter including us stably exist in this world…!